When maintaining a food safety management system, SQF practitioners are responsible for continuous improvement. They know that Corrective and Preventative Action (CAPA) and Root Cause Analysis (RCA) are quality management tools that drive continuous improvement and improve SQF Audit Scores. When deviations from SQF requirements are identified, a systematic process must be followed to determine the root cause of a deviation so that corrections can be applied to prevent a recurrence. CAPA and RCA are proactive activities that support the PDCA approach (Plan Do Check Act). Your team can improve your SQF audit scores by applying consistently the principles of CAPA.
Root cause analysis (RCA) and Corrective and Preventative Action (CAPA) are known quality management processes that can help you improve your SQF compliance even more. We break down both in our blog. Here are our Key Tips to improve SQF Audit Scores with CAPA.
Defining CAPA and RCA
According to SQF Code edition 9 (Food Manufacturing), Root Cause Analysis (or RCA) is a method of problem-solving to identify and resolve the core issue(s) that cause a non-conformity, deviation, or other adverse food safety or quality event.
Corrections are defined as actions to eliminate a detected nonconformity.
Corrective Actions are actions to eliminate the cause of a detected nonconformity identified at a food safety audit, a deviation identified at a quality audit, or other undesirable situations and to prevent a recurrence. This is also referred to as “corrective and preventative action (CAPA).”
Both CAPA and RCA are processes that continuous improvement teams use to correct issues and avoid costly recalls and customer complaints. The tools are used to identify the root cause of serious customer complaints, HACCP plan failures, crises like recalls and critical GMP deviations. In addition, CAPA is a process employed to fix internal and external audit non-conformities. A disciplined approach of using CAPA and RCA after a facility audit can dramatically improve SQF audit scores.
Apply the 4 steps of CAPA and RCA to improve SQF audit scores.
The first key tip to improve SQF Audit Scores is to apply the four steps of CAPA and RCA.
- Identify and define the problem
Step 1 of CAPA describes the scope of the food safety or quality issue which will require an investigation of the problem by the SQF team.
This process is evidence-based and necessitates objective evidence that the food safety or quality management system is non-compliant. A review of customer complaint logs, internal audit reports or foreign material logs can provide sources of objective evidence that can be used to initiate the CAPA process.
- Evaluate and Review the Impact on the Site
The intent of Step 2 is to appraise the negative outcome and assess how the lack of compliance will have on the business. During this phase, the team describes the food safety hazard or quality threat, identifies the impacted product lots, and narrows down the timeframe for non-compliance. CAPA team members discuss the process(es) that were likely not performed according to written procedures. They discuss the immediate corrections that the facility took (for example, equipment emergency repairs, placing defective lots on hold, and retraining employees). The team will also discuss the possible impact on the consumer and the need to recall food at this stage.
- Investigate the Nonconformity
The team assembles the necessary expertise to tackle the problem. Step 3 of CAPA analyzes the contributing factors leading to non-conformance. The stakeholders will confirm the sequence of events by listing the dates and times that the product was held and what customer complaints were received. A review of process monitoring logs may provide a sense of when the issue appeared. A record review backed up by employee interviews may confirm when the last compliant batch of products was released to the market.
The investigation may also consider product (or equipment) specifications and all ingredients/packaging materials listed on the bill of materials.
Lastly, the team may identify whether immediate corrections were successful in fixing the problem (did the facility receive customer complaints after immediate corrections were implemented?).
- Analyze the Root Cause of the Problem
The RCA phase looks beyond the immediate problem or crisis management. It seeks to uncover the underlying cause(s) of the problem and forms the link to corrective and preventive actions (CAPA). Root cause analysis deploys various tools and techniques to collect data and facilitate the investigation process.
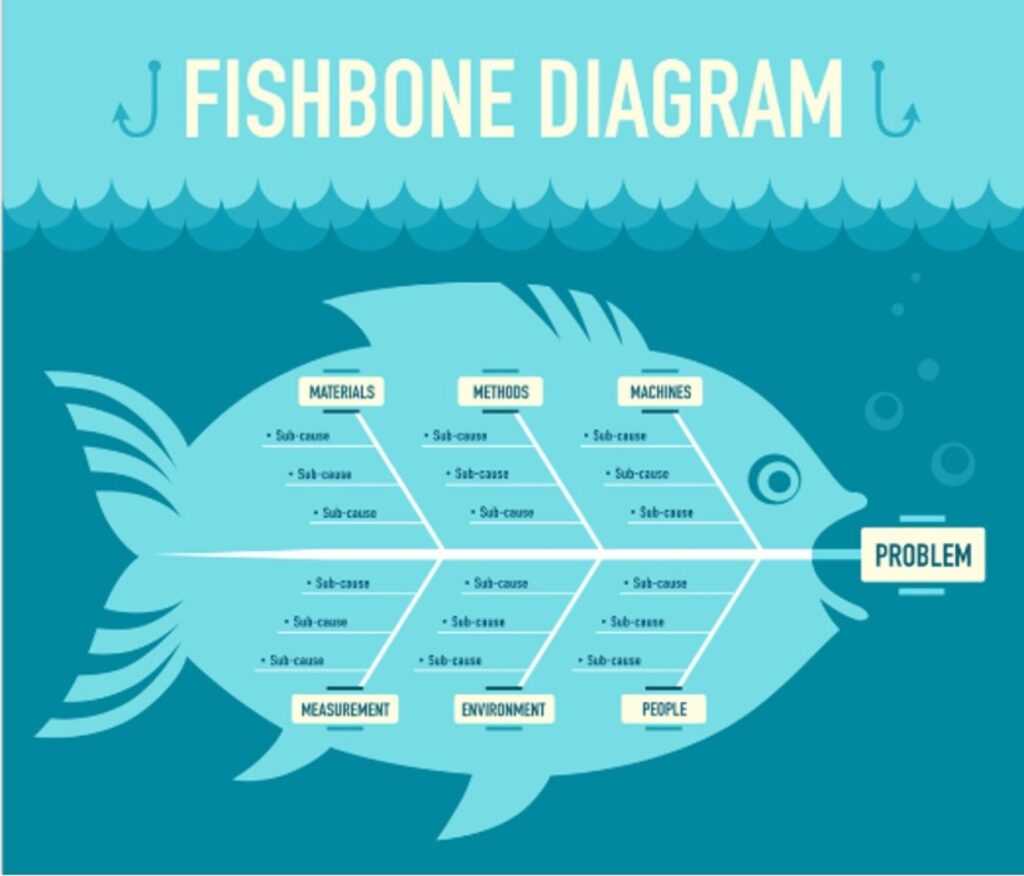
CAPA teams may use graphs (Pareto charts, cause and effect diagrams, process reviews and interviews). Techniques may include The 5 Why’s Method, a Fishbone or 6M Diagram (featured in this article), or a Cause and Effect Analysis (Refer to the ASQ website for more information).
These RCA techniques are important tools for SQF practitioners. The value of these techniques is to focus on nonconformity causes, not the symptoms of deviations. It captures and trends the collective knowledge and experience of the site which then supports the decision-making process for continuous improvement. This is how RCA and CAPA improve your SQF audit scores.
Fishbone Diagram or “6M” Diagram
The second key tip to improve SQF Audit Scores is to use a systematic method to perform Root Cause Analysis or “RCA”. The Ishikawa Diagram, also known as Cause-and-Effect Diagram, 6M or Fishbone Diagram is one of the quality tools for continuous improvement. This technique comprises 6 categories of possible root causes. Each category is considered by the CAPA team, and possible sub-causes are identified and prioritized. Examples of questions the team should consider when using this method are as follows:
 Source: Shutterstock, 2022
Source: Shutterstock, 2022
Manpower (“People”)
- Does the workforce including supervisory personnel receive sufficient support/resources and training?
- Do employees understand the job function? Do they receive training matching their job descriptions? Are they qualified and motivated to perform the job function?
- Is the environment conducive to their performance?
- Have there been any changes in human resources (seasonal or contracted employees, staff turnover, lack of leadership/supervision)?
Methods
- Is the process outlined in a Standard Operating Procedure?
- Is the process regularly audited?
- Do criteria exist for process performance?
- Are employees aware of process objectives and do they know how to make process adjustments?
- Does the team utilize Statistical Process Control (SPC) or trending to verify the effectiveness of the process?
- Has the process changed recently?
Materials
- Has the safety of the raw material been assessed (Country of origin/vendor, risk assessment)?
- Does the material meet specifications (shelf life, micro, purity)?
- Can food packaging affect material specifications (tamper-evident packaging)?
- Can external factors affect specifications (moisture, temperature, oxygen)?
- Has there been a change in material specifications?
Machines
- Is equipment preventive maintenance performed regularly?
- Are machines and other equipment out-of-date? Has equipment failed in the past?
- Are there external factors influencing performance? Is the equipment able to achieve the right specification/outcome?
- Have employees commented on the performance of the equipment?
- Have there been recent physical changes to machinery?
- How is the equipment cleaned? What tools are used to clean the equipment?
Mother Nature (“Environment”)
- Is the manufacturing environment controlled (ex: site’s pest control)?
- Is the environment clean (EMP program, sanitation, air quality)?
- Are there changes in process conditions at different times of the day or the year?
- Was the site recently upgraded (construction)? Have external conditions changed?
- Are the environmental conditions inspected? Do they meet GMP?
Measurements
- Is the frequency of quality checks and other analytical measurements sufficient to validate safety or quality?
- Are vendor COAs verified by internal or external testing?
- Are instruments and sensors calibrated by competent personnel?
- Are analytical methods recognized? (Is proficiency testing conducted annually?)
- Any recent changes to the calibration program?
Develop an Action Plan and Assign Responsibilities
The next key tip to improve SQF Audit Scores is to develop an Action Plan and Assign Responsibilities to the team. Specific tasks are assigned to competent personnel who understand their food safety responsibilities. As with other SQF programs, Corrective Action Reports or known as “CAR” are maintained. Timelines for verification and completion are determined by consensus but should not exceed 30 days. This phase also addresses structural changes to the site or equipment, employee training and process/document revisions. The plan is communicated to all departments and members of the internal audit team. One can improve SQF Audit Scores by ensuring that corrective actions are fully implemented to prevent recurrence of a problem. SQF Auditors do not like repeat non-conformities. It signals to them that your CAPA process is weak.
Review and Assess the Effectiveness of the Corrective Action Plan
The last key tip to improve SQF Audit Scores is to review the effectiveness of your CAPA program. This last phase involves executing the action plan and assessing the effectiveness of corrective actions. Did the team identify the root cause correctly? Is the root cause eliminated? Did modifications to process and equipment result in new issues that must be addressed?
Once the plan is implemented and modifications documented, the CAPA team monitors the new process to ensure that the root cause has been addressed. Once the manufacturing process has been returned to compliance, the CAR reports may be closed. At this stage, all documents must be dated and signed by appropriate, authorized personnel.
In summary, CAPA applied to food safety management allows the site to initiate, evaluate, assign, monitor, review, and approve actions in a formal manner. The CAPA workflow is based on the review of objective evidence. Prioritization of corrective actions is based on food safety risk outcomes. Interesting in reading more resources on CAPA, check our blog on the subject.
The content of this blog on CAPA and RCA includes information contained in the SQFI Advanced SQF Practitioner Course which is one of the accredited courses taught by Karine Lawrence (Certified SQF Trainer and Consultant).
Sirocco Food & Wine Consulting provides food safety training and consulting services. Looking to support your lead and back-up SQF practitioners? Contact us to schedule a free consultation.
References:
SQF Code Edition 9 Food Manufacturing





